Instructions for use
1. Instructions for Use
These instructions are intended to help you use the Evela Health Platform safely and as intended. All content is written in clear and understandable language.
The instructions are updated regularly when new versions or features are released. Please always use the most recent version.
If you have questions or need support, you can contact our support team at any time: [support@evela.health]
These instructions for use meet the requirements of Regulation (EU) 2017/745 (MDR).
1.1 Trade Name and Version
The trade name of this product is: Evela Health Platform
These instructions apply to software version: 1.02
Issue date: 30.07.2025
1.2 Manufacturer Information
Manufacturer:
Evela Health GmbH
Chausseestraße 58D
10115 Berlin, Germany
Email: [support@evela.health]
Website: [www.evela.health]
In case of serious incidents related to the use of this product, please contact both the manufacturer and the relevant national authority without delay.
1.3 Electronic Provision (eIFU)
These instructions are provided exclusively in electronic form (eIFU).
The latest version is always available online at: [www.app.evela.health]
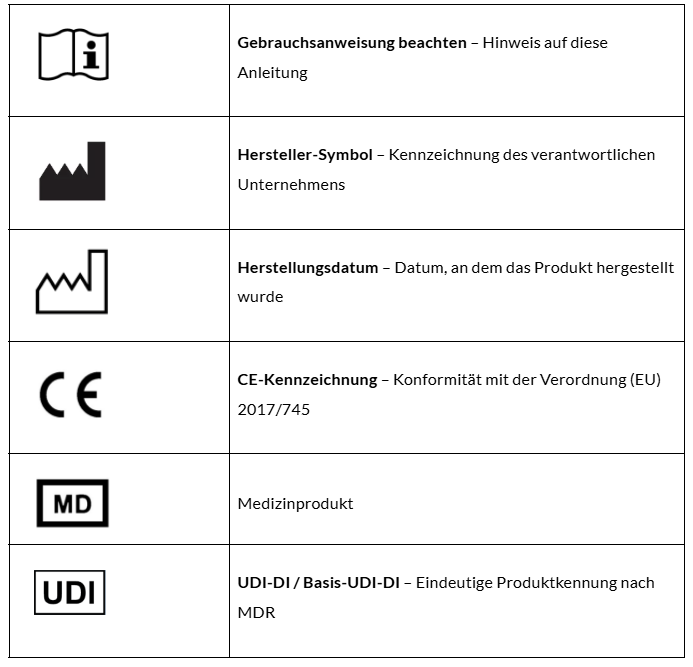
1.4 Labels and Symbols
The Evela Health Platform carries the legally required labels:
CE mark in accordance with MDR
MD symbol for medical device
Manufacturer symbol with name and address
Manufacturing date symbol with software release date
UDI-DI (GTIN): 04262550850009
Basic UDI-DI (GMN): 426255085EV-MP-1T
An overview of the symbols used with explanations can be found in Chapter 9 “Symbols and Legal Notices.”
2. Intended Purpose
The Evela Health Platform is a browser-based software application for women in midlife, particularly during the menopausal transition (perimenopause and menopause). The platform follows a multimodal, lifestyle-oriented approach, considering various aspects of daily life, including nutrition, exercise and physical activity, sleep, mental health and resilience, sexuality and relationships, skin and hair, prevention, and the use of medications and supplements. All content is evidence-based and aligned with the current state of science.
The platform supports you in:
accessing information and general content on menopause, health, and lifestyle topics (e.g., nutrition, exercise, sleep, skin and hair, prevention, resilience),
recording your symptoms using the *Menopause Rating Scale (MRS II)** and tracking them over time, as well as documenting personal habits and priorities,
preparing for consultations with experts through structured questionnaires (Health Assessment),
scheduling and conducting consultations with experts.
The Evela Health Platform itself does not provide diagnoses, interpret inputs, or give automated recommendations. Individual recommendations are provided solely in consultations with the connected experts.
The software does not replace medical examinations or treatments. All content is general in nature and not tailored to individual persons.
Use of the platform is entirely self-directed by you as the user. Medical decisions are always made by physicians or healthcare professionals and are not an integral part of the Evela Health Platform. Recommendations for medical examinations can only be made by human experts, not by the software itself.
3. Product Description
The Evela Health Platform was specifically developed for women in midlife. It serves as a tool for structured self-reflection, symptom tracking during menopause, and preparation for expert consultations. The content is based on a multimodal, lifestyle-oriented approach covering areas such as nutrition, exercise, sleep, mental health, sexuality, skin and hair, prevention, and the use of medications and supplements.
After successful registration (see Chapter 4), several sections are available to actively reflect on and manage your health and wellbeing. A detailed description of how to use the functions can be found in Chapter 5 “Main Functions.”
3.1 Overview of Functions
The Evela Health Platform consists of four main sections:
1. Home with Symptom Diary (MRS II)
Track symptoms using the scientifically validated Menopause Rating Scale (MRS II).
Repeated entries help observe changes over time and recognize patterns.
2. Evela Campus
Articles, videos, and audios covering menopause, nutrition, exercise, sleep, mental health, sexuality, skin and hair, prevention, and supplements.
Content is evidence-based and may be personalized based on your input.
3. My Data
Collects and visualizes entries from the symptom diary and questionnaires.
Provides an overview of changes over time to support consultations.
4. Experts
Book and conduct lifestyle consultations online.
Experts provide individual recommendations based on your input.
3.2 Premium Area
The paid Premium area offers expanded options, including:
Health Assessment questionnaire covering multiple life areas,
video consultations with experts,
a personalized health plan summarizing recommendations from the consultation,
access to all premium content in the Evela Campus.
If necessary, cooperating physicians can also be involved, e.g., for prescriptions.
3.3 Technical Requirements
To ensure smooth use:
The platform is browser-based (no installation or app download required).
A current internet browser (e.g., Chrome, Safari, Edge, Firefox) is needed.
A stable internet connection is required.
You need a user account (valid email + secure password).
Optimized for desktop, tablet, and smartphone.
Video consultations use secure, approved communication services.
Accessibility aids (e.g., screen readers, zoom) are supported, though some content may be partially limited.
4. Registration and First Steps
To use the Evela Health Platform, you first need to create a personal account. This ensures your data is stored securely and accessible only to you. The registration process has three steps: creating the account, setting a secure password, and agreeing to the necessary consents.
4.1 Creating an Account
Enter your email address (used as username and for confirmation).
Set and confirm a password. Requirements:
at least 8 characters,
at least one number,
passwords must match.
You’ll receive a confirmation email with a six-digit code. Enter it to activate your account.
4.2 Password and Security Notes
Your password protects your personal data.
Do not share it with anyone.
Forgotten passwords can be reset via the platform.
4.3 Privacy and Consent
Before using the platform, you must agree to:
Terms of Use (mandatory),
Health Data Processing (mandatory for features like the symptom diary),
Analysis & Personalization** (to tailor content),
Research (optional):** pseudonymized data may be used for scientific purposes.
More information is available in the Privacy Policy: [https://app.evela.health/privacy]
Data is processed according to GDPR and MDR Annex I, Chapter III (technical and organizational measures).
5. Main Functions
The platform offers features for symptom tracking, health information, and consultation preparation. Premium features provide additional detail and personalization.
5.1 Home & Symptom Diary – based on MRS II, with graphical overviews of trends.
5.2 Evela Campus – knowledge hub with articles, videos, audios.
5.3 My Data – structured overviews, trends, and (in Premium) Health Assessment results.
5.4 Experts – secure video consultations with experts.
5.5 Premium Health Assessment – detailed questionnaire, personalized health plan.
6. Safety Information
6.1 General Notes
Informational and reflective use only.
No diagnosis or medical recommendations.
Keep login details safe and use trusted devices.
6.2 Contraindications
Not suitable in emergencies (e.g., chest pain, severe bleeding, acute mental crisis).
→ Call emergency services (112 in Germany) or see a doctor.
6.3 Warnings
Not a replacement for medical care.
Consult professionals if symptoms worsen or persist.
6.4 Risks of Misuse
Misinterpreted symptoms, delayed medical care, misunderstood content, data breaches.
7. Incident Reporting
Report any problems immediately.
Manufacturer contact:
Evela Health GmbH
Email: [support@evela.health](mailto:support@evela.health)
Website: [www.evela.health](http://www.evela.health)
Authorities:
Serious incidents must also be reported to national authorities. In Germany: BfArM – Federal Institute for Drugs and Medical Devices ([www.bfarm.de](http://www.bfarm.de)).
8. Data Protection & Security
Only necessary data is processed (email, symptom diary, questionnaires, consultations).
Processing complies with GDPR.
Security: TLS encryption, access restrictions, logs, backups.
Video consultations via approved, secure providers.
Privacy Policy: [https://www.evela.health/de/privacy-policy](https://www.evela.health/de/privacy-policy)
9. Legal Provisions, Notices, and Symbols
Classified as a "Class I medical device" under EU MDR, Rule 11.
Intended use: information and consultation preparation (not diagnosis/treatment).
Complies with MDR and applicable standards (ISO 13485, ISO 14971, IEC 62304, IEC 82304-1, IEC 62366-1, ISO 20417, ISO 15223-1, EU 207/2012).
Note: Intended only for adult users. Safety instructions in Chapter 6. Incident reporting in Chapter 7.
Symbols used are explained in Chapter 9.